SOLVOMET/SIM2 KU Leuven researchers have developed a process to remove trace concentrations of cadmium, zinc and manganese from simulated diluted mine waters via foam separation. The work, which was published in the Journal of Sustainable Metallurgy, was performed in the framework of the H2020 MSCA-ETN SULTAN project for the remediation and reprocessing of sulfidic mining waste sites.
As environmental regulations are becoming stricter, new techniques must be developed for the removal of trace concentrations of heavy metals from mineral processing effluents. Foam separation techniques are an interesting alternative to more conventional processes such as ion exchange because of their efficiency to treat dilute aqueous streams.
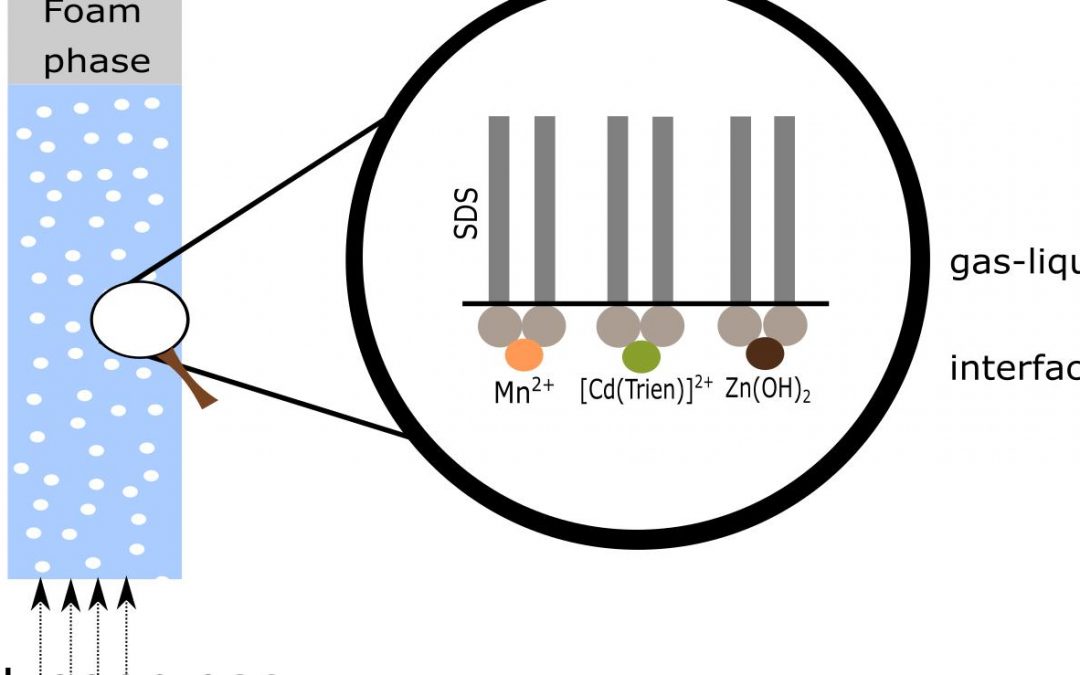
In this paper, the simultaneous removal of Cd2+, Mn2+, and Zn2+ from dilute aqueous solutions was investigated by using sodium dodecyl sulfate as collector and triethylenetetramine as auxiliary ligand via a series of batch-mode flotation experiments.
Experimental results showed that Cd2+, Mn2+, and Zn2+ can be completely removed in one step under the following conditions: pH = 9.50, flotation time = 120 min, auxiliary concentration 0.1 mmol L−1, collector-to-metals molar ratio 2:1, ethanol concentration 0.5% (v/v), and a nitrogen gas flowrate set at 25 mL min−1. An excess in auxiliary ligand concentration yielded to low removal efficiency.
The modeled speciation of the examined system suggested that the metals are separated from the bulk solution to the foam phase via a combination of ion flotation and precipitate flotation.
Full reference paper
Xanthopoulos, P., Binnemans, K. Removal of Cadmium, Zinc, and Manganese from Dilute Aqueous Solutions by Foam Separation. J. Sustain. Metall. (2021). https://doi.org/10.1007/s40831-020-00322-2
Acknowledgements
This project has received funding from the European Union's EU Framework Program for Research and Innovation H2020 under Grant Agreement No 812580.